Abstract
Background: Burkitt lymphoma and diffuse large B-cell lymphoma (DLBCL) are the most common types of mature B-cell lymphoma (MBL) in children. A majority (90%-95%) of children with these malignancies are cured with initial chemoimmunotherapy (CIT), leaving a small portion of patients with relapsed or refractory (R/R) disease who have a poor prognosis (Cairo et al, J Clin Oncol 2012). R/R MBL is often treated with aggressive CIT regimens such as R-VICI or R-ICE, but a high, unmet need remains (Woessmann et al, Blood 2020). Long-term survival is observed in approximately 20% to 30% of pediatric cases of R/R MBL, with better long-term survival seen in those who attain complete response (CR) with reinduction CIT and reach consolidation with stem cell therapy. Patients with partial response (PR) after reinduction CIT may undergo stem cell transplant but typically have lower long-term survival rates than those experiencing CR (Burkhardt et al, Cancers 2021). Long-term survival or cure in patients with R/R MBL requires an effective therapy that results in CR before stem cell transplant. Epcoritamab is a bispecific antibody that binds CD3 on T cells and CD20 on B cells, inducing potent and selective T-cell-mediated killing of malignant CD20+ B cells. The phase 1/2 EPCORE NHL-1 study (NCT03625037) of epcoritamab monotherapy in adult patients with R/R B-cell non-Hodgkin lymphoma established the recommended phase 2 dose (RP2D) and demonstrated safety and promising efficacy, including deep and durable responses (overall response rate [ORR], 63%; CR rate, 39%; preliminary median duration of response [DOR], 12 months) in a population with highly refractory large B-cell lymphoma (n=157) (Thieblemont et al, EHA 2022). Because favorable safety and efficacy data were observed in adults, it is reasonable to investigate use of epcoritamab in children with R/R MBL who fail to achieve CR with reinduction CIT or are unable to receive further consolidation with stem cell therapy after CIT.
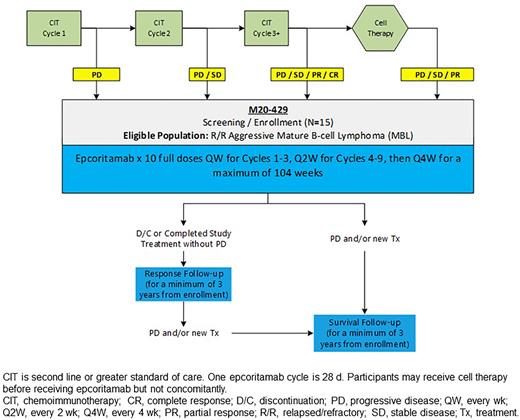
Study Design and Methods: EPCORE Peds-1 is a single-arm, open-label, phase 1b study (NCT05206357) evaluating the safety, pharmacokinetics, pharmacodynamics preliminary efficacy, and immunogenicity of epcoritamab monotherapy in pediatric and young adult patients with R/R Burkitt lymphoma, Burkitt-like lymphoma/leukemia, DLBCL, or other aggressive CD20+ MBL. Primary endpoints are safety and tolerability, including incidence and severity of cytokine release syndrome, immune cell-associated neurotoxicity syndrome, and clinical tumor lysis syndrome, as well as pharmacokinetic parameters of maximum observed concentration and area under the concentration-time curve from time 0 to last measurable concentration within the dosing interval. Secondary endpoints include CR per International Pediatric Non-Hodgkin Lymphoma response criteria, event-free survival, overall survival, initiation of stem cell transplant/chimeric antigen receptor T-cell therapy, ORR, DOR, duration of CR, and immunogenicity. Exploratory endpoints include the monitoring of pharmacodynamic markers such as cytokines and immunophenotyping of B and T cells. At least 15 patients will be enrolled with a maximum age of 25 years (for those with Burkitt lymphoma), with at least 12 patients 1 year of age or older but younger than 18 years who are evaluable for primary endpoint assessment. Eligibility criteria require that patients have not reached remission with reinduction CIT or are ineligible for further consolidation with cell therapy and have acceptable performance status (Lansky, Karnofsky, or Eastern Cooperative Oncology Group, depending on age). Epcoritamab will be subcutaneously administered using step-up dosing (priming and intermediate doses on days 1 and 8 of cycle 1 [28 days/cycle], followed by 10 weekly full doses from day 15 of cycle 1 through cycle 3; Figure). Additional full doses will be administered every 2 weeks for cycles 4 to 9 and every 4 weeks for cycles 10 and beyond for a maximum of 2 years of treatment. Epcoritamab priming-, intermediate-, and full-dose regimens will be based on weight categories; patients weighing at least 40 kg will receive the adult RP2D. Disease status will be assessed by CT/MRI/PET prior to epcoritamab administration on day 1 and at a minimum at weeks 6, 12, 24, 36, and 48 and at 18 and 24 months. Patients will be followed for at least 3 years after enrollment. Enrollment is ongoing in North America, Asia, Europe, and Australia.
Disclosures
Cairo:Nektar: Consultancy; Jazz: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; Servier: Consultancy, Speakers Bureau; Omeros: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Sanofi: Speakers Bureau; Sobi: Speakers Bureau; AbbVie: Consultancy. Rocco:AbbVie: Current Employment, Current equity holder in publicly-traded company; Johnson & Johnson: Current equity holder in publicly-traded company. Bernard:AbbVie: Current Employment, Current equity holder in publicly-traded company. Siddani:AbbVie: Current Employment, Current equity holder in publicly-traded company. Parikh:AbbVie: Current Employment, Current equity holder in publicly-traded company. Elliott:Genmab: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal